Cultivated meat is expensive, but open-source bioprocessing is helping to change that. By sharing tools, methods, and data freely, the industry is tackling high costs in areas like growth media, bioreactor design, and cell line development. Key highlights include:
- Growth media costs have dropped significantly, with food-grade alternatives replacing pharmaceutical-grade components. For example, a new medium costs as little as £0.52 per litre.
- Open-source bioreactor designs reduce equipment expenses by eliminating royalty fees and improving efficiency.
- Shared research into cell lines and continuous production methods is improving scalability and reducing waste.
These efforts aim to bring cultivated meat closer to price parity with conventional meat - projected to cost as little as £4.30 per kilogramme by 2030. Open collaboration is key to achieving this shift, making cultivated meat more accessible for everyday consumers.
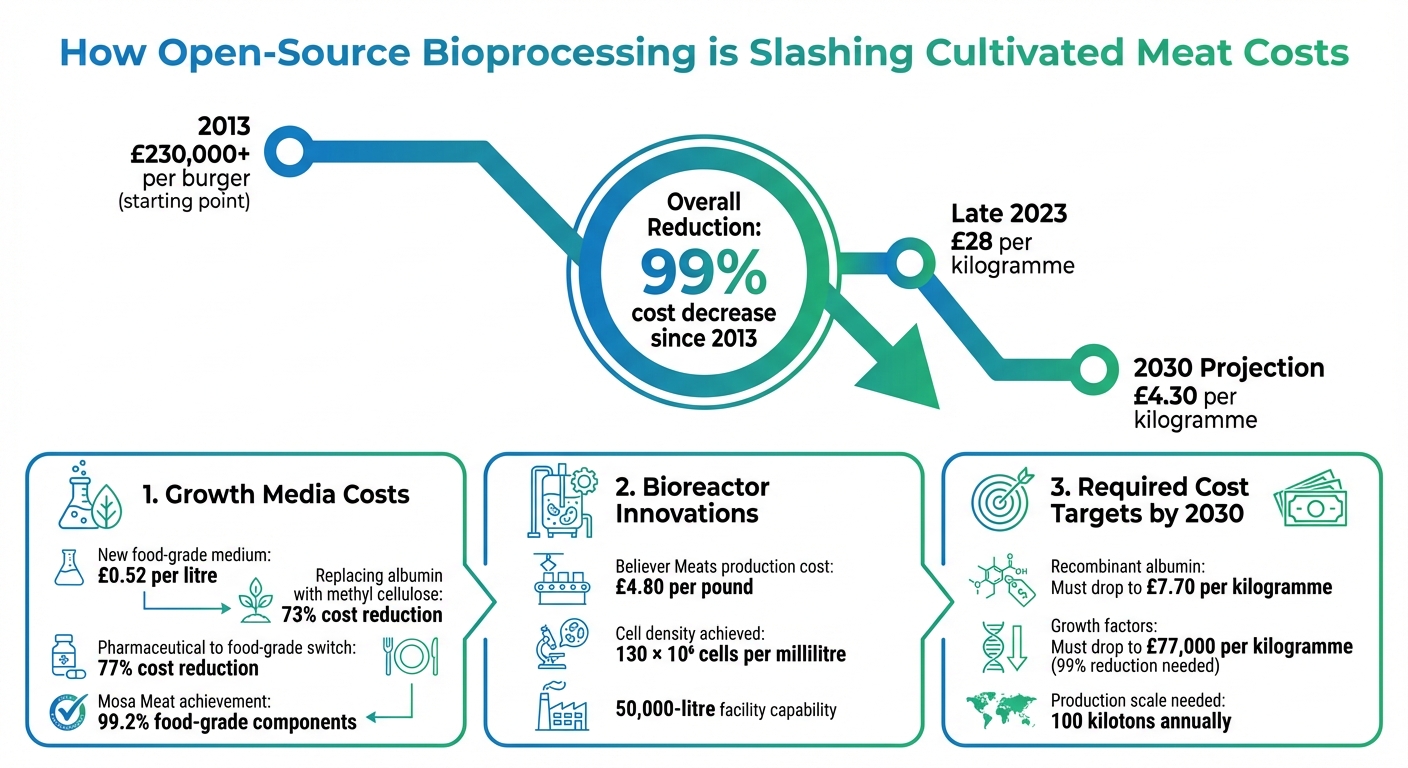
Cultivated Meat Cost Reduction Timeline: From £230,000 to £4.30 per kg (2013-2030)
What Is Open-Source Bioprocessing?
The Basics of Open-Source Bioprocessing
Open-source bioprocessing is all about making research, designs, and technical data freely available. In the Cultivated Meat industry, this means sharing critical resources like bioreactor performance models, growth media formulations, and techno-economic analyses. These tools help predict costs and yields, giving the industry a shared starting point to tackle production challenges.
Instead of companies pouring millions into developing the same basic tools, open-source projects provide a communal foundation - think of it as a "Wikipedia for bioprocessing." This shared knowledge base can be refined and expanded, saving time and resources while driving innovation.
A great example of this came in June 2024, when Simon Hubbard from Upstream Applied Science Ltd launched an open-access bioreactor performance modelling workflow. Using computational fluid dynamics and Bayesian optimisation, the project predicted yields for 20,000-litre and 42,000-litre stirred tank reactors. Hubbard made the entire workflow available on GitHub, giving the industry a chance to optimise bioreactor designs and operations without needing costly proprietary software [2].
"There is, at present, no openly available modelling workflow that enables yield prediction or optimisation for given bioreactor architecture, geometry and operational inputs." – Simon Hubbard, Upstream Applied Science Ltd [2]
By sharing resources like this, open-source initiatives not only cut development costs but also encourage collaboration across the industry.
Why Open-Source Collaboration Works
One of the biggest benefits of open-source collaboration is that it eliminates redundant research. If one team cracks a technical problem - like reducing ammonia build-up in bioreactors or improving oxygen supply - sharing that solution means others don’t have to spend time and money solving the same issue.
Open-source projects also make it easier for newcomers to join the industry. By offering free access to formulations and production processes, these initiatives lower the financial barriers for entrepreneurs, especially in emerging markets [4]. For example, open-access computational models allow developers to predict yields and experiment with different bioreactor designs before committing to costly construction [2].
The pharmaceutical industry provides a useful parallel. It already uses stirred tank bioreactors at scales of up to 20,000 litres for producing therapeutic proteins [3]. Open-source bioprocessing enables Cultivated Meat producers to adapt these well-established systems while addressing their unique challenges - like the fact that muscle cells are more sensitive to shear forces than the suspension cells used in pharma.
3 Ways Open-Source Bioprocessing Reduces Production Costs
Cutting Costs in Growth Media
Growth media, the nutrient-rich liquid essential for cell cultivation, is one of the biggest expenses in producing Cultivated Meat. Open-source projects have made a real difference here by sharing formulations like Beefy-9, B8, and Essential 8, eliminating the need for costly licensing fees [5][6].
Another game-changer is the move from pharmaceutical-grade ingredients to food-grade alternatives. While pharmaceutical-grade materials undergo extensive purification processes that inflate costs, open research has shown that food-grade options can perform just as effectively for growing meat cells [6][7]. For instance, in August 2024, researchers at Believer Meats showcased a continuous manufacturing process using an animal-component-free medium priced at just £0.52 per litre (about $0.63) [8]. This is a massive reduction compared to traditional media formulations.
Additionally, advancements in bioreactor designs play a significant role in further reducing costs.
Lower-Cost Bioreactor Innovations
Beyond affordable growth media, open-source bioreactor designs are driving down production expenses even further. Bioreactors - large tanks used for cell cultivation - are traditionally costly to produce and maintain. Open-source designs remove the financial burden of royalty fees and allow manufacturers to tailor systems to their specific requirements [5]. In October 2023, New Harvest, IRNAS, and the University of Maribor released a modular, perfusion-based bioreactor design on GitHub. This design integrates seamlessly with standard lab equipment, cutting capital costs and enabling programmable nutrient circulation for growing 3D tissue cultures [5].
"Open-source licensing means the hardware, firmware, software and documentation can be used without paying a royalty." – New Harvest [5]
These designs also support continuous manufacturing, which significantly improves efficiency. Using perfusion technology, Believer Meats achieved cell densities of up to 130 × 10⁶ cells per millilitre, surpassing traditional batch methods. Their research estimates that a 50,000-litre facility could produce cultivated chicken at a cost of £4.80 per pound (around $6.20), making it comparable to organic chicken prices [8].
Advancing Cell Lines and Production Techniques
Open-source collaboration has also accelerated progress in cell line development and production methods. Shared research into stable, high-yield cell sources - such as spontaneously immortalised chicken fibroblasts - has provided the industry with reliable cell lines that grow without the need for animal serum [8].
Digital tools like computational modelling have further streamlined production by allowing producers to optimise designs virtually, reducing risks and costs.
Perhaps the most impactful improvement is the shift from batch processing to continuous production. Traditional batch methods require frequent interruptions for cleaning and re-seeding, which slows down production. Continuous perfusion, on the other hand, enables multiple harvests over a 20+ day period from a single inoculation, dramatically improving both efficiency and economic feasibility [8].
Lab-Grown Meat Could Feed the Planet | TIME
sbb-itb-c323ed3
Open-Source Projects Lowering Cultivated Meat Costs
Efforts to reduce costs in cultivated meat production have taken a major leap forward, thanks to open-source projects focused on improving media formulation and optimising processes.
Shared Media Development Projects
Collaborative initiatives have made significant strides in cutting the cost of growth media. For example, in 2022, Andrew Stout and his team at Tufts University introduced Beefy-9, an open-source serum-free medium designed to sustain long-term bovine satellite cell culture without relying on costly Fetal Bovine Serum. By incorporating recombinant albumin and fine-tuning growth factor concentrations, they developed a formula that is freely accessible. Later research (2024/2025) revealed that replacing human serum albumin with food-grade methyl cellulose could reduce medium costs by up to 73%. This is a game-changer, as albumin in the widely used B9 formulation costs around £19 per litre (approximately $24.56), making up more than half of the total media expense [9].
Another noteworthy achievement came from Mosa Meat, working alongside Nutreco. They managed to replace 99.2% of their basal cell feed with food-grade components, all while maintaining cell growth rates comparable to pharmaceutical-grade media [6]. These examples highlight how collaboration across the industry can directly lower the production costs of cultivated meat.
In addition to these technical breakthroughs, organisations like the Good Food Institute have provided critical cost-modelling tools to guide further progress.
Good Food Institute Open-Source Platforms
The Good Food Institute (GFI) has been instrumental in equipping the industry with data and research to drive cost reductions. In 2020, Dr Liz Specht, GFI's Lead Scientist, published a pivotal analysis showing that switching pharmaceutical-grade basal media components for bulk food-grade alternatives could lower basal media costs by 77% [6][10]. This work offered a clear path towards achieving price parity with conventional meat, reinforcing the earlier examples of cost-saving innovations.
GFI's research also pinpointed areas where further cost reductions are essential. For instance, their analysis projected that by 2030, recombinant albumin would account for 96.6% of the total production volume of proteins required, while growth factors, though representing just 0.02% of the volume, remain the most expensive components. To make cultivated meat more affordable, albumin production costs need to drop to roughly £7.70 per kilogramme (around $10), while growth factors should reach about £77,000 per kilogramme (approximately $100,000) [10].
"The manufacturing capacity for growth factors and recombinant proteins would need to scale rapidly alongside the cultivated meat industry... which comprise just 0.1% to 0.56% of the projected global meat demand expected in 2030." – Good Food Institute [10]
These insights and collaborative efforts underscore the potential of open-source projects to reshape the economics of cultivated meat production, bringing it closer to mainstream viability.
Cost Projections for Cultivated Meat by 2030
Expected Cost Reductions by 2030
The economics of Cultivated Meat are on the brink of a major shift, largely driven by open-source bioprocessing. Since 2013, the cost of production has plummeted by an astonishing 99%. Back then, producing a single burger cost over £230,000, but by late 2023, the price had dropped to around £28 per kilogramme [11][12]. With ongoing advancements, experts predict that by 2030, Cultivated Meat could cost as little as £4.30 per kilogramme [11].
To hit these targets, the industry will need to scale up production to approximately 100 kilotons annually. Another key factor will be improving the efficiency of growth media, aiming for usage levels between 8 and 13 litres per kilogramme of Cultivated Meat [10].
That said, there are still significant hurdles to overcome. For instance, the cost of recombinant albumin must decrease to about £7.70 per kilogramme, while growth factors need to drop to roughly £77,000 per kilogramme - a staggering 99% reduction from current biopharmaceutical production costs [10]. As highlighted by the Good Food Institute:
"99% cost reduction may be required for some recombinant proteins compared to how they are currently produced for the biopharmaceutical industry" [10].
These ambitious projections underline the immense progress required to bring costs closer to conventional meat.
Current vs. Projected Production Costs
When comparing today’s costs with future estimates, the scale of innovation needed becomes clear. Predictions for 2030 vary, with some suggesting prices could fall to £4.30 per kilogramme [11], while others lean towards a more cautious estimate of £28 per kilogramme [10]. Despite these reductions, Cultivated Meat still costs more than traditional meat. Achieving true price parity will rely on continued open-source collaboration and further advancements in production scaling, metabolic efficiency, and bioreactor technology.
Conclusion
Main Points
Open-source bioprocessing is tackling some of the most expensive hurdles in Cultivated Meat production. By focusing on collaborative efforts, the industry is finding practical ways to make Cultivated Meat more affordable and accessible. For instance, researchers have discovered ways to replace costly recombinant proteins with plant-based alternatives like rapeseed protein and shift from pharmaceutical-grade to food-grade ingredients, which can be up to 100 times less expensive [1]. Open-source bioreactor workflows are also helping to mitigate financial risks by improving predictions for large-scale production yields.
The results are promising. To make Cultivated Meat commercially viable, the cost of growth media needs to drop significantly - from hundreds of pounds per litre to around £0.77 per litre. Open-source initiatives are playing a crucial role in this transformation, promoting the use of plant protein hydrolysates and refining cell lines that can thrive in nutrient-minimal environments. As David Block from UC Davis explains:
"To make cultivated meat commercially viable, that number [growth media cost] is probably going to have to be $1 per liter or less - so orders of magnitude lower." [1]
Notable examples highlight this progress. Tufts University has demonstrated how plant proteins can replace recombinant albumin, while open-access bioreactor workflows are advancing cost-saving innovations. In 2023, a new culture medium using rapeseed protein instead of recombinant albumin set a benchmark for cost reduction [1][7]. Similarly, in June 2024, Simon Hubbard shared an open-access bioreactor performance workflow on GitHub, equipping the industry with tools to enhance facility designs [2]. These shared resources showcase how open collaboration is driving the affordability of Cultivated Meat.
How You Can Stay Informed
As the industry continues to evolve, staying informed is essential. The Good Food Institute offers valuable insights through its "State of the Industry" reports and its open Solutions Database [13][14]. For more technical updates, journals like npj Science of Food provide open-access reviews on strategies for reducing costs and improving growth media [15][16].
For a broader understanding of this emerging technology, visit Cultivated Meat Shop (https://cultivatedmeat.co.uk). The platform offers accessible content on product types, availability, and the science behind Cultivated Meat, helping you explore how this innovation could shape the future of food. It’s an exciting opportunity to learn about a world where sustainable meat is grown from cells, not animals.
FAQs
How does open-source bioprocessing help reduce the cost of cultivated meat?
Open-source bioprocessing is playing a key role in making cultivated meat more affordable. By offering accessible, low-cost tools and shared knowledge, it supports both research and production efforts. Take open-source modular bioreactors, for instance. These systems provide an economical alternative for researchers to design and refine production equipment, sidestepping the hefty costs tied to proprietary setups. Their flexible designs not only lower entry barriers but also accelerate innovation within the industry.
On top of that, open-source initiatives foster collaboration on cost-saving measures, such as enhancing growth media formulations and scaling up production methods. Sharing progress in areas like sourcing affordable ingredients and improving cell growth efficiency helps cut operational costs. This collective effort is paving the way for cultivated meat to become more accessible and commercially feasible for consumers in the not-too-distant future.
How do food-grade alternatives help reduce the cost of growth media in cultivated meat production?
Growth media play a major role in the cost of producing cultivated meat, with traditional options often relying on pricey components like fetal bovine serum (FBS). However, the use of food-grade alternatives has emerged as a game-changer, making production far more affordable and easier to scale.
Switching out expensive ingredients for food-grade substitutes - such as serum albumins and recombinant proteins - has led to dramatic cost reductions, sometimes slashing expenses by up to 100 times. These advancements don't just cut costs; they also bring cultivated meat closer to matching the price of conventional meat. This progress is paving the way for cultivated meat to become a more accessible and appealing option for consumers across the UK and beyond.
How do open-source bioreactor designs help reduce the cost of cultivated meat?
Open-source bioreactor designs are proving to be a game-changer in making cultivated meat production more affordable. By offering accessible and flexible production systems, these designs allow manufacturers to tailor bioreactors specifically for cultivated meat, focusing on areas like boosting cell growth rates and using resources more efficiently. This customisation helps cut down on production costs significantly.
Another major advantage of open-source models is how they promote collaboration and innovation. Researchers and industry professionals can work together to develop advanced bioreactor technologies faster. This includes designing systems that support higher cell densities, conserve energy, and generate less waste. These improvements not only streamline the production process but also make cultivated meat a more cost-effective option, bringing it closer to being an affordable choice for consumers.













