Scaling bioreactors is the key to making cultivated meat more affordable and accessible. Over the past decade, the cost of cultivated meat has dropped from £1.8 million per kilogram in 2013 to £49 per kilogram today, thanks to advancements in large-scale production. By the end of 2026, global production is projected to reach 125,000 tonnes, with prices potentially falling to as low as £1.52 per kilogram.
This progress depends on solving technical challenges like oxygen transfer, heat dissipation, and nutrient distribution in larger bioreactors. Different bioreactor designs - stirred-tank, airlift, perfusion, and hollow-fibre - offer unique solutions but come with trade-offs in scalability and efficiency. New technologies, such as media recycling, single-use systems, and real-time monitoring, are helping reduce production costs further.
For consumers, this means cultivated meat could soon match or even undercut the price of conventional meat, with large-scale bioreactors producing enough to feed 75,000 people annually. This shift also reduces resource use, making cultivated meat a viable option for both affordability and sustainability.
The Challenges of Scaling Bioreactors
Technical Barriers to Scale-Up
Moving from lab-scale experiments to industrial bioreactors comes with a host of technical hurdles. One major issue is oxygen transfer. As bioreactor size increases, oxygen solubility becomes a bottleneck. Larger vessels struggle with prolonged mixing times, which can create uneven oxygen distribution. This leads to some cells being deprived of oxygen while others are oversaturated, disrupting the delicate balance required for cell growth [8].
Heat management is another significant challenge. The larger the bioreactor, the smaller the surface-area-to-volume ratio becomes [8]. Animal cells generate metabolic heat, and while a small lab flask can naturally dissipate this heat, a massive 100,000-litre vessel needs advanced cooling systems to maintain the narrow temperature range that cells can tolerate [2, 9].
These cells also have a fragile structure. Unlike bacteria or yeast, animal cells lack a protective cell wall, making them vulnerable to mechanical forces [2]. Large-scale reactors require high-speed mixing, but this creates turbulence that can damage the cells. Bioprocess engineer Muhammad Arshad Chaudhry highlights the complexity of scaling bioreactors:
"Bioreactor scaling is not trivial; it is a difficult and complex task that requires a delicate balance between equipment design and operational capabilities... to provide similar hydrodynamic and mass-transport conditions" [8].
Nutrient distribution also becomes uneven in larger systems. Poor circulation leads to "stagnant zones" where essential nutrients like glucose are depleted, while harmful byproducts such as ammonia and lactic acid build up [2, 9]. Taller reactors introduce yet another issue: the increased liquid height raises pressure at the bottom, making it harder to remove carbon dioxide, which can become toxic at high concentrations [8]. On top of all this, the risk of contamination skyrockets. A single contaminated batch in a 50,000-litre reactor could result in a devastating financial loss [2, 6].
All these factors combine to reduce efficiency and drive up production costs.
How Scale-Up Challenges Affect Cost
The technical difficulties of scaling bioreactors don't just complicate production - they also significantly increase costs. For example, poor oxygen transfer and uneven nutrient distribution slow cell growth, reducing overall yield. This directly raises the cost per kilogramme of cultivated meat [6, 9]. The need for high-grade stainless steel equipment to ensure sterility adds further to the expense, with these capital costs ultimately reflected in product prices [3, 6].
Industry analyst David Humbird explains the limitations succinctly:
"Low growth rate, metabolic inefficiency, catabolite and CO₂ inhibition, and bubble-induced cell damage will all limit practical bioreactor volume and attainable cell density" [5].
These constraints make it difficult for current production methods to match the efficiency and cost-effectiveness of traditional meat farming.
The financial stakes are enormous. Back in 2013, the cost of producing cultivated meat was an eye-watering £1.8 million per kilogramme. Today, that figure has dropped to around £49 per kilogramme [4]. While this is a huge improvement, achieving true affordability requires overcoming the technical barriers to scaling. Economic models suggest that switching to integrated continuous processing could cut capital and operating expenses by 55% over a decade compared to batch processing [2]. However, these savings hinge on solving the persistent technical challenges that come with scaling up.
Dr. Marianne Ellis: Designing large-scale bioreactors and bioprocesses for cultivated meat
How Bioreactor Types Influence Scaling
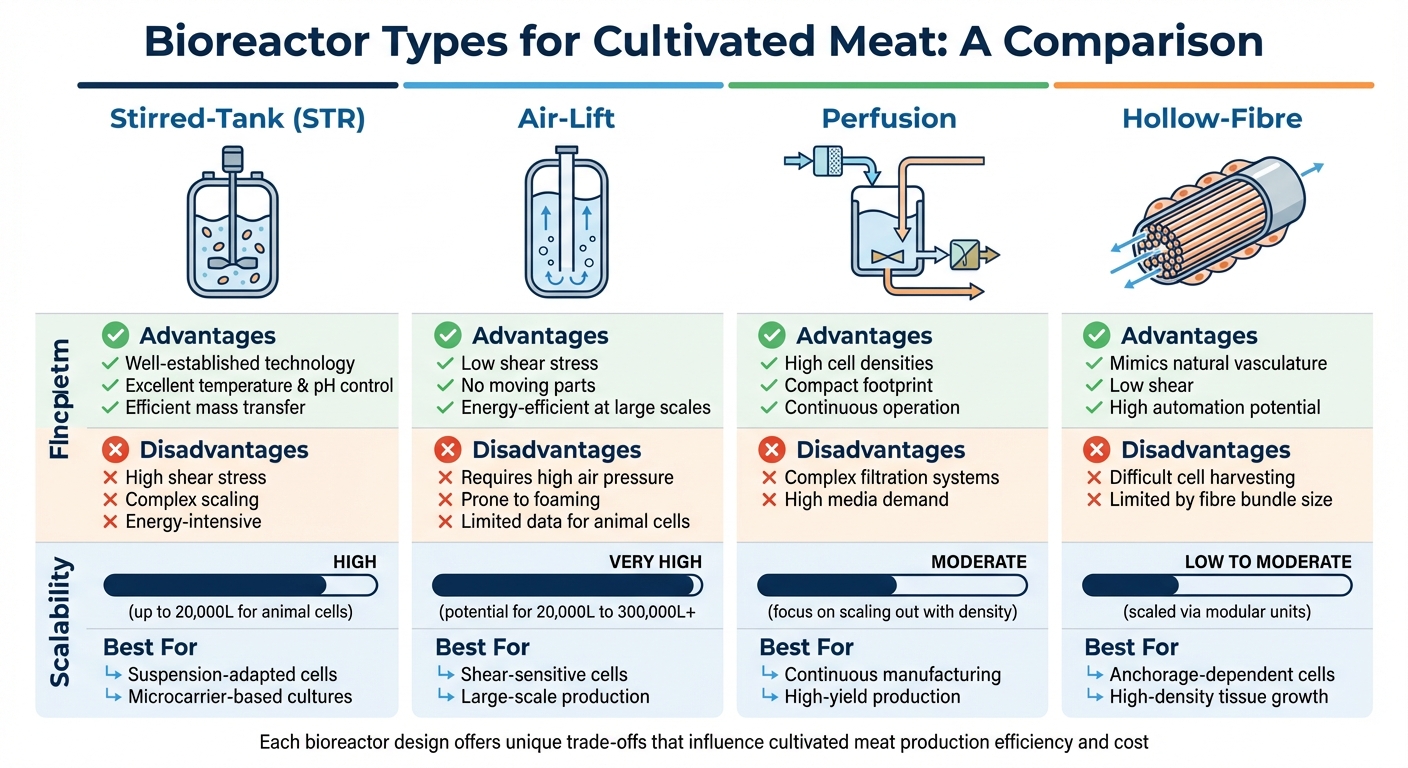
Bioreactor Types Comparison for Cultivated Meat Production
The type of bioreactor used in production plays a critical role in shaping efficiency, costs, and, ultimately, the price consumers pay. Different reactor designs come with unique challenges and benefits, which directly impact how well they handle scaling up production. Understanding these differences is crucial for overcoming scale-up hurdles and reducing costs.
Stirred-tank bioreactors are the industry standard, originally developed for biopharmaceutical manufacturing. These reactors use mechanical impellers to mix the cell culture medium and maintain oxygen levels. They’re effective for volumes as large as 20,000 litres [2][6]. However, the impellers create shear forces that can damage delicate animal cells, which lack the protective cell walls found in bacteria or yeast [2]. As Cathy Ye, Director of the Oxford Centre for Tissue Engineering and Bioprocessing, highlights:
"A major technical issue is controlling the disruptive shear forces on large volumes of fragile mammalian cells, while maintaining the necessary stirring of the cells in their sustaining fluid" [9].
Airlift bioreactors offer a promising alternative for large-scale production. Instead of mechanical mixing, they use gas injection in a 'draft tube' design to circulate the medium with minimal shear stress. These reactors are incredibly scalable - one airlift reactor designed for microbial growth held 1,500,000 litres [2]. For cultivated meat, a theoretical 300,000-litre airlift reactor could support cell densities of 2×10⁸ cells/mL, enough to feed 75,000 people annually [2]. Despite their potential, airlift reactors have limited historical data for animal cell use [2].
Perfusion bioreactors take a different approach by focusing on high cell densities in smaller volumes. They achieve this through continuous media exchange, which allows cells to grow while waste is removed. Economic models suggest that this method can reduce capital and operating costs by 55% over a decade compared to traditional batch processing [2]. However, these systems are complex, requiring advanced filtration to retain cells while removing waste.
Hollow-fibre systems mimic the vascular networks found in living organisms. They use thousands of semi-permeable capillary tubes, with cells growing in the space around the fibres while nutrients flow through them. This setup can achieve exceptionally high cell densities of 10⁸ to 10⁹ cells/mL [2][7]. However, scaling these systems involves adding modular units, which limits their use for mass production.
Bioreactor Types Compared
Here’s a breakdown of the key differences:
| Bioreactor Type | Advantages | Disadvantages | Scalability | Best Applications |
|---|---|---|---|---|
| Stirred-Tank (STR) | Well-established; excellent temperature and pH control; efficient mass transfer [2][10] | High shear stress; complex scaling; energy-intensive [2][8] | High (up to 20,000L for animal cells) [2] | Suspension-adapted cells; microcarrier-based cultures [2] |
| Air-Lift | Low shear stress; no moving parts; energy-efficient at large scales [2] | Requires high air pressure; prone to foaming; limited data for animal cells [2] | Very High (potential for >20,000L to 300,000L+) [2] | Shear-sensitive cells; large-scale production [2] |
| Perfusion | High cell densities; compact footprint; continuous operation [2][7] | Complex filtration systems; high media demand [2][7] | Moderate (focus on "scaling out" with density) [2] | Continuous manufacturing; high-yield production [2] |
| Hollow-Fibre | Mimics natural vasculature; low shear; high automation potential [2][7] | Difficult cell harvesting; limited by fibre bundle size [2][7] | Low to Moderate (scaled via modular units) [7] | Anchorage-dependent cells; high-density tissue growth [2] |
Each bioreactor design offers unique advantages and trade-offs that influence how cultivated meat can be scaled efficiently. Stirred tanks are reliable but face physical limitations at larger volumes. Airlift reactors present opportunities for massive scaling but require more development for animal cells. Perfusion systems offer efficiency in smaller spaces but come with operational challenges. Meanwhile, hollow-fibre systems excel in achieving high densities but are limited in scalability. These differences will play a key role in making cultivated meat more accessible to consumers.
sbb-itb-c323ed3
Solutions to Bioreactor Scaling Challenges
The cultivated meat industry is pushing boundaries to make bioreactors more efficient and affordable, paving the way for large-scale production that balances cost and performance.
Technical Advances
New technologies are addressing the hurdles of scaling up production. One major shift involves using food-grade materials instead of expensive pharmaceutical-grade equipment. For instance, swapping 316 stainless steel for 304 stainless steel and opting for chlorine dioxide gas sterilisation instead of steam can significantly cut capital costs [1][3]. Unlike drug manufacturing, cultivated meat production doesn’t require extreme sterilisation levels, making these changes both practical and economical.
Another breakthrough is media recycling, which tackles the high costs of growth media. Techniques like tangential flow filtration and cell retention devices allow companies to reuse media while filtering out waste [1][3]. This ensures nutrients remain abundant without the need to replace the entire medium constantly.
Real-time monitoring systems are also transforming the industry. Equipped with advanced sensors, these systems use AI and machine learning to optimise conditions such as pH, oxygen, and temperature. This reduces batch failures and ensures consistency. As Matt McNulty, a GFI Research Fellow, explains:
"Designing fit-for-purpose bioreactor technologies, which have been specifically engineered to meet the needs of the cultivated meat industry, has the potential to reduce bioprocessing costs" [1].
Another promising approach is single-use technology, where disposable bioreactor bags eliminate the need for cleaning and sterilisation. While these bags are currently expensive, efforts are underway to develop more cost-effective, food-safe versions [1][2]. Additionally, process intensification - such as high-density cell banking and combining cultivation and differentiation in a single vessel - offers ways to streamline production [1].
These advancements, combined with smarter production strategies, are shaping the future of cultivated meat manufacturing.
Scaling-Out vs. Scaling-Up
To make cultivated meat more accessible and cost-efficient, the industry is exploring two key scaling strategies. Scaling-up involves building enormous bioreactors, often exceeding 20,000 litres. This approach offers significant economies of scale, lowering capital and labour costs per unit of production [1][2]. However, larger vessels come with engineering challenges, such as managing shear stress and heat dissipation.
On the other hand, scaling-out focuses on using multiple smaller bioreactors, typically ranging from 100 to 1,000 litres [2]. This modular strategy allows for quicker market entry, avoiding the complexities of massive bioreactors and enabling greater automation. As GFI points out:
"Scaling out approaches may provide a more reasonable short-term path to market for cultivated meat products... however, production volumes at these scales will likely fail to meet the large demands for global meat consumption" [2].
To further optimise costs, many companies are turning to integrated continuous processing, which can reduce capital and operating expenses by up to 55% over a decade compared to traditional batch processing [2]. A hybrid strategy is emerging, where scale-out facilities address immediate local demand while scale-up plants are developed for larger-scale production. These combined efforts are crucial for making cultivated meat more affordable and accessible to consumers worldwide.
What This Means for Consumers
The evolution in bioreactor design and the resulting cost reductions are starting to bring tangible benefits to everyday consumers, making cultivated meat more accessible and affordable.
Affordability and Accessibility
Advances in bioreactor systems have drastically cut costs, with prices dropping from millions to around £50 per kilogramme. Even better, forecasts suggest this could fall further to as low as £1.50 per kilogramme [4]. These savings are the result of scaling up production and refining processes.
Scaling up is a game-changer here. For instance, a massive 262,000-litre airlift bioreactor can produce cultivated meat at an estimated £13 per kilogramme, compared to approximately £27 per kilogramme from smaller 42,000-litre stirred-tank systems [11]. Research indicates that consumer acceptance could increase by as much as 55% if prices align with traditional meat [4]. This progress hints that it won’t be long before cultivated meat finds its way into UK supermarkets and butcher shops.
Environmental Benefits
Beyond cost, these innovations also address environmental concerns. Scaled-up bioreactors significantly reduce the resources needed for meat production, including energy and land, offering a more sustainable alternative.
Airlift reactors stand out for their efficiency, especially in volumes over 20,000 litres. Their simple design - with no moving parts - uses far less energy than traditional stirred-tank systems [2][11]. When paired with advanced media recycling systems, these reactors make large-scale cultivated meat production a greener option compared to conventional livestock farming [3][11]. Industry projections estimate an output of around 125,000 tonnes by the close of 2026 [3].
Role of Platforms like Cultivated Meat Shop
As these breakthroughs unfold, educating consumers becomes crucial. Platforms like Cultivated Meat Shop play a key role in bridging the gap between lab innovations and the products that will soon hit the shelves.
Conclusion
Scaling bioreactors is at the heart of making cultivated meat a practical alternative to traditional meat. Thanks to recent advancements, production costs have plummeted - from millions of pounds to approximately £50 per kilogramme - with forecasts suggesting prices could drop to as low as £1.50 [4]. These reductions open the door to greater affordability and accessibility for consumers across the UK.
Progress in tackling challenges like shear stress, oxygen transfer, and cell density in large-scale bioreactors is paving the way for mass production. For instance, a 300,000-litre bioreactor has the potential to feed 75,000 people annually [2]. As Kristala Prather, Department Executive Officer of Chemical Engineering at MIT, aptly puts it:
"While the science for making cultivated meat products might be ready, the cost of doing so has to meet the parameters for a feasible business model" [9].
The adoption of integrated continuous processing has also proven to be a game-changer, slashing capital and operational costs by up to 55% over a decade [2].
For UK consumers, these developments signal a turning point - moving cultivated meat from experimental labs to supermarket shelves. With industry output expected to hit 125,000 tonnes by the end of 2026 [3], and consumer acceptance increasing by 55% when prices fall below those of conventional meat [4], the momentum is undeniable. On top of that, the environmental advantages - such as reducing land and water use by up to 98% [12] - make the case for cultivated meat even stronger.
As production continues to scale, platforms like Cultivated Meat Shop will play a crucial role in helping UK consumers navigate this rapidly evolving market. Scaling bioreactors isn’t just about engineering; it’s about creating a future where meat is more sustainable, affordable, and accessible for everyone.
FAQs
Why does scaling bioreactors affect the price of cultivated meat?
Scaling up bioreactors is no small feat, and it plays a big role in determining the cost of cultivated meat. Larger bioreactors come with their own set of hurdles, such as increased shear stress, longer mixing times, and higher energy consumption. These factors collectively push up production expenses. On top of that, many existing bioreactor designs simply aren’t tailored for large-scale cultivated meat production, which limits their efficiency and keeps costs high.
Addressing these issues is crucial to making cultivated meat more budget-friendly for consumers. As advancements enhance the performance and scalability of bioreactors, production will become more economical, inching cultivated meat closer to becoming a regular feature on dinner tables.
How does large-scale bioreactor production benefit the environment?
Large-scale bioreactor production presents a promising shift away from traditional livestock farming, bringing with it a host of environmental advantages. By producing cultivated meat directly from cells, this method can significantly cut greenhouse gas emissions, use far less water, and require much smaller amounts of land for food production.
Beyond its environmental perks, this approach also addresses global food security challenges. By moving away from resource-heavy farming practices, it offers a way to meet growing food demands more efficiently. As bioreactor technology continues to advance, cultivated meat could emerge as a more sustainable and widely available alternative to conventional meat.
Why is bioreactor design important for scaling cultivated meat production?
Bioreactors are at the heart of scaling cultivated meat from small lab experiments to full-scale industrial production. The design of these systems significantly influences production costs, scalability, and how efficiently cells grow.
Take continuous stirred-tank reactors (CSTRs), for instance. These are popular because they can handle large volumes and provide excellent oxygen transfer. But there’s a catch - intense mixing in CSTRs can damage fragile animal cells and lead to higher energy use as the scale increases. On the other hand, setups like wave bioreactors and single-use vessels are gentler on cells and cut down on cleaning costs. These features make them great for smaller-scale production, but their limited size and challenges with nutrient distribution can be barriers to scaling up.
Then there are more specialised options like air-lift reactors and packed-bed systems. These designs aim to lower energy demands or support higher cell densities. However, they often need fine-tuning to address issues like mass transfer limitations or fouling. In the end, the choice of bioreactor comes down to finding the right balance between efficiency, cost, and scalability. Getting this balance right is a critical step towards making cultivated meat more affordable and accessible for consumers.













